Product Pipeline
Monepantel is an approved veterinary product for the treatment of nematode infestations in sheep. In mammalian cells, monepantel is a potent inhibitor of the mTOR pathway. The mTOR pathway plays a central role in cell growth and proliferation of cancer cells. In degenerating neurons the mTOR pathway regulates autophagic flux and the recycling of cellular macromolecules.
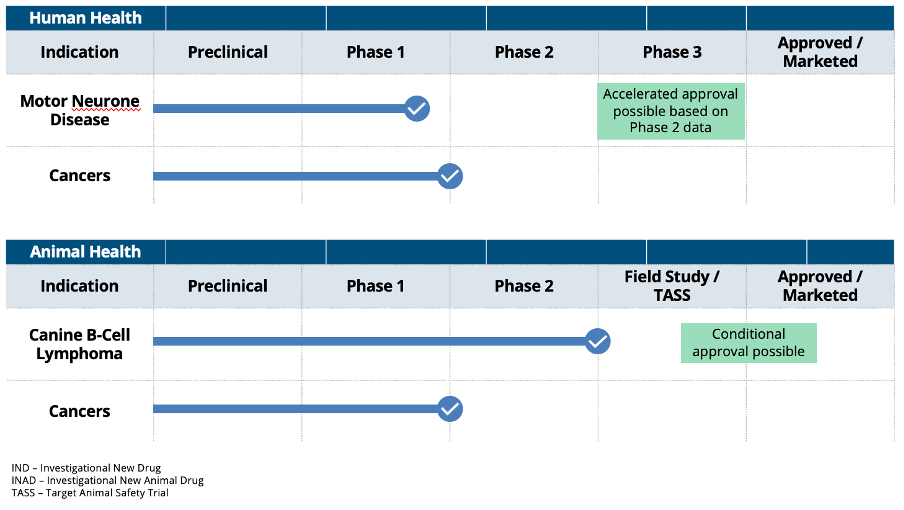
PharmAust’s Therapeutic Pipeline
Monepantel is an approved veterinary product being repurposed for the treatment for cancer in dogs and motor neurone disease in humans. Pipeline synergies allow commercial infrastructure to be leveraged across animal and human health applications.